The COVID 19 virus has the world in turmoil. It has managed to significantly diminish the human and economic resources of countries. Each day witnesses a new rise in cases and number of deaths. While everybody is eagerly waiting for life to go back to normal, the end of this pandemic looks quite uncertain. Just out of curiosity, how do you think this will end?
One solution can be achieving herd immunity. Herd immunity can be reached if 70-90% of the population is exposed to the virus and recovers from the disease to develop antibodies against future infection. This would break the chain of transmission and provide indirect protection to those who are not immune to the virus. However, with a fatality rate of 0.5-1%, achieving herd immunity would entail a high human cost. Also, the immunity gained after recovering from the virus is not permanent, meaning that in a few months after recovery, you’ll again be at a risk of getting infected. This makes herd immunity an impractical and far fetched approach. This is why, as acknowledged by WHO, CDC and MoHFW, a vaccine is the only answer to triumph over COVID-19.
In layman’s terms, a vaccine or immunization is a medicine that prevents a particular disease. It is important to know that it is not a curative but a preventive measure. The concept behind a vaccine is to stimulate an antibody memory response without producing an illness. Thus, it mimics the virus it protects against, causing a fight-back response from the immune system. This way, when the actual virus enters the body, the antibodies are ready to tackle it. But is there a vaccine for every disease? There’s no guarantee. For example, a vaccine against the Ebola virus was developed in five years whereas the vaccine against AIDS still does not exist. However, due to the urgency of the situation, the vaccine against COVID19 is being developed at a much faster rate than any other vaccine. The entire world has invested all of its time and resources into developing a safe and effective vaccine for this pandemic.
Development of a vaccine is a long and stringent process. The vaccine passes through a number of layered trials before getting approved for general use. Since the risks associated with an unsafe vaccine are high, it is dangerous to haste the development procedure by compromising on the efficacy of the product. Such strict measures are imperative to ensure no side effects in a vaccine, since side effects of an unsafe vaccine can potentially be more dangerous than the disease it is supposed to protect against. Currently, more than 170 teams of researchers are racing to develop a safe and effective vaccine. Despite the cumulative efforts, a cloud of uncertainty floats over the future of COVID 19 vaccine. The below table will give you a brief understanding of the various stages of vaccine development and also a quick update on the number of candidates at each stage:
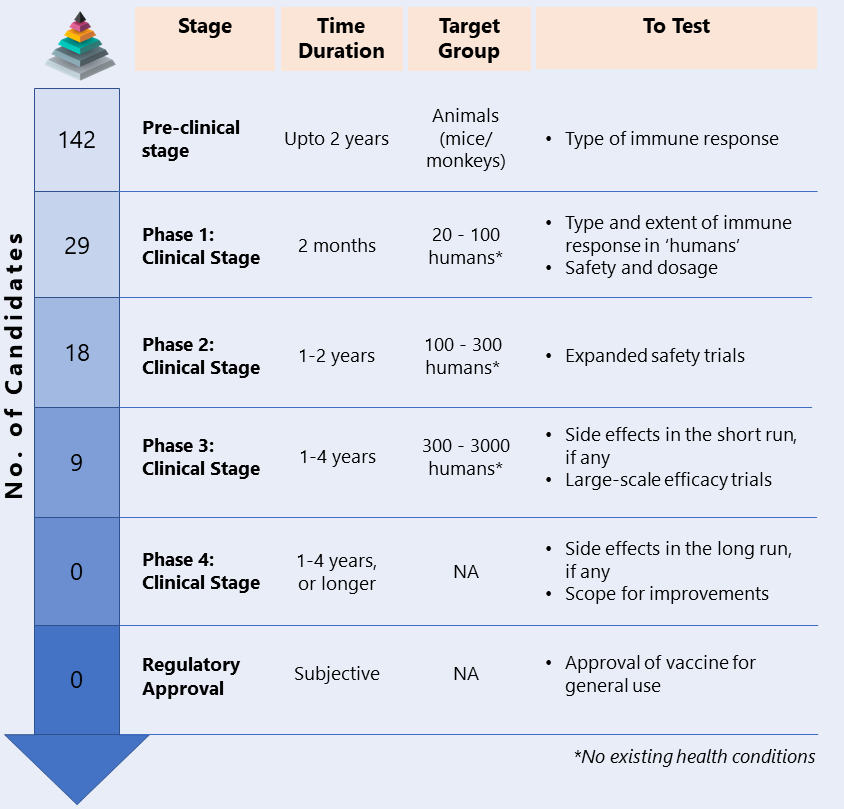
Once a vaccine clears the benchmarking process and receives approval from the respective authorities, it is ready for large scale production and subsequent distribution. Manufacturers with large scale production capacity get the license to produce the vaccines in bulk. This licence includes stringent measures on quality control. Once the vaccine is produced, it is distributed as per dissemination protocol set by the government, generally in the following order of priority:
Table of Contents
Frontline workers –
medical staff, public administration officials etc.
Vulnerable sections –
children, senior citizens, persons with comorbidities
General public
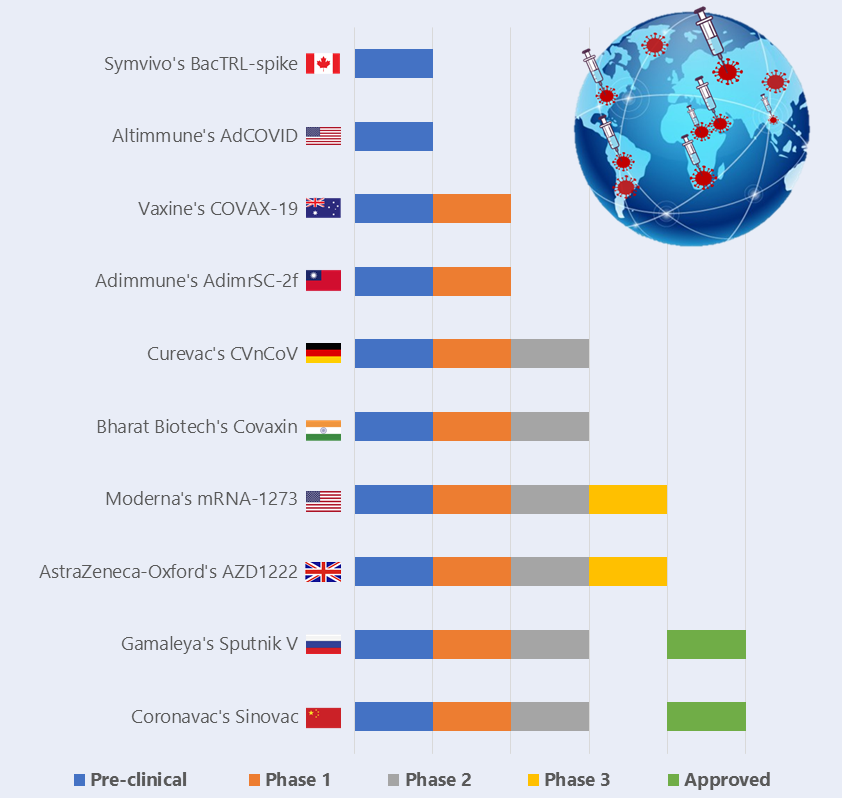
A vaccine may also get “emergency use authorization” before getting final approval when the exigencies of the pandemic situation calls for it. Two such vaccines, China’s Sinovac and Russia’s Sputnik V, have been launched without clearing the Phase 3 stage in the vaccine development process. This has raised some serious concerns about their safety. A recent study has reported that 1 in every 7 individuals who have been administered the Sputnik V vaccine have reported mild side-effects like body pain, sore throat and headache. However, Russia has planned a global Phase 3 study of its vaccine, involving 45000 volunteers. Russia has proposed to include India as a part of this global bridge trial and the country has also signed a pact with Dr Reddy’s Laboratory, a leading multinational pharmaceutical company based in India, for distribution of the vaccine upon successful completion of Phase 3 trials and registration with Indian regulators.
The Serum Institute of India, based in Pune, is the world’s largest vaccine producer. It is in a collaborative tie with the University of Oxford to conduct the Phase 3 human trials of the ‘Covishield’ vaccine, the candidate of the AstraZeneca-Oxford University. It is expected to be the first vaccine to be released in India and will be produced in large quantities by the Serum Institute. Although the global trials of the aforementioned vaccine were temporarily halted due to a case of spinal inflammation in one of the volunteers, it has received the green flag to continue testing after the required investigation. The Serum Institute has declared that the price of this vaccine will be capped at Rs 225 per dose and will be released in Q1 2021. This was also confirmed by Dr Harsh Vardhan, the Union Health Minister of India. According to Adar Poonawala, CEO of the Serum Institute of India, the task of buying and distribution of vaccines to immunize the entire nation will be Rs. 80,000 crores assuming 2 doses per person. This poses a concerning challenge to the MoHFW and PMO, if the government were to bear the cost of this immunization programme.
While leading COVID-19 vaccine candidates are advancing at an exceptional rate to advanced stages of clinical development, many uncertainties remain, given the complexity of the vaccine development process and the limited data available to date. Despite this, the hope persists that a viable, safe and efficient vaccine will be developed soon. Until then, it is our responsibility to maintain social distancing and appropriate hygiene. Step out of your home only if absolutely necessary and wear a mask when you do. Let’s pledge to fight this invisible enemy responsibly.
Stay Healthy ! Stay Protected !
A version of this post first appeared on LinkedIn. We welcome your feedback in the comments section below. This is an initiative by Healthysure as part of our #COVIDCare series.
Follow us on our LinkedIn Page to catch regular updates on COVID-19 and Healthcare topics.
About Healthysure
Healthysure is an employee-focused InsurTech platform, founded by an IIM-B alum. We aim to redefine employee health protection in India through our unified health insurance offering that clubs the benefits of corporate and retail health insurance into a combined comprehensive product, bundled with other health and wellness offerings. To make an enquiry, write to us on ********@*********re.in “>ge********@*********re.in
******
This article is authored by Laksh Maheshwari with inputs from Dr. Amrit Jha (MBBS, MS Ortho) & Dr. Darshan Sheth (MBBS, MHA).
About Laksh Maheshwari
A tech enthusiast with a knack for word play. I’m a keen follower of social and political affairs in and around the world. I’m passionate about learning and exploring new domains. If it’s a holiday, you can find me trekking on a nearby hill.
Laksh is a content writer at Healthysure.





